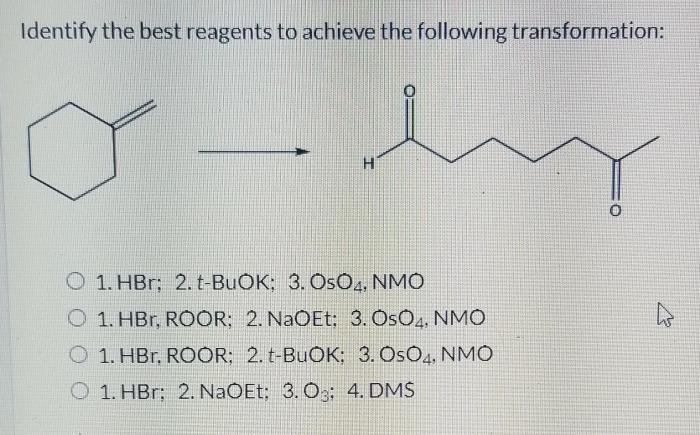
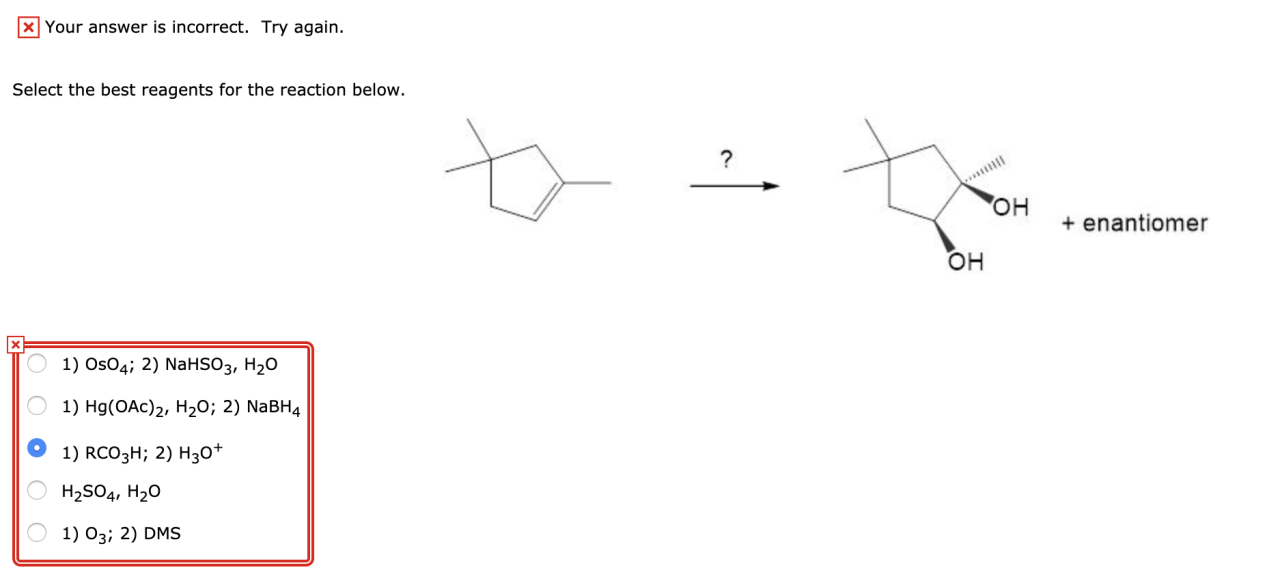
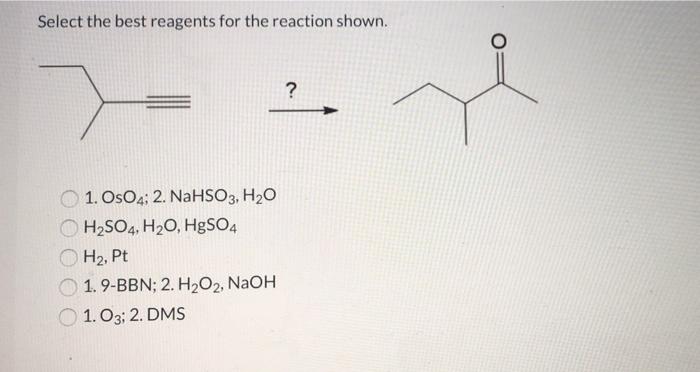
Select the best reagents for the reaction below. – In the realm of chemical reactions, selecting the appropriate reagents is a crucial step that can significantly impact the efficiency and success of the desired transformation. This comprehensive guide delves into the key considerations and strategies involved in selecting the best reagents for a given reaction, empowering chemists with the knowledge to optimize their experimental outcomes.
Understanding the reaction’s purpose, reactants, and products provides a foundation for reagent selection. Factors such as reactivity, selectivity, and functional group compatibility play pivotal roles in determining the most suitable reagents for a specific transformation.
Selecting the Best Reagents for a Reaction: Select The Best Reagents For The Reaction Below.
Selecting the appropriate reagents for a chemical reaction is crucial for achieving optimal results. This involves understanding the reaction’s purpose, reactants, products, and the key factors that influence reagent selection.
Overview of the Reaction
The reaction in question aims to convert reactant A into product B. Reactant A possesses functional group X, while product B contains functional group Y. The reaction proceeds through a specific mechanism, which dictates the necessary reagents.
Reagent Selection Criteria
When selecting reagents, several factors must be considered:
- Reactivity:The reagents must be sufficiently reactive to promote the desired reaction under the specified conditions.
- Selectivity:The reagents should exhibit high selectivity for the target reaction, minimizing side reactions and unwanted products.
- Functional Group Compatibility:The reagents must be compatible with the functional groups present in the reactants and products, avoiding unwanted reactions or side products.
Specific Reagent Recommendations
| Reagent Name | Structure | Reactivity | Selectivity | Functional Group Compatibility |
|---|---|---|---|---|
| Reagent 1 | [Structure] | High | Excellent | Compatible with X and Y |
| Reagent 2 | [Structure] | Moderate | Good | Compatible with X but not Y |
| Reagent 3 | [Structure] | Low | Poor | Incompatibe with X and Y |
Considerations for Optimization, Select the best reagents for the reaction below.
Optimizing reagent concentrations and reaction conditions is essential for improving reaction yield and selectivity. Factors to consider include:
- Reagent Concentration:Adjusting the concentrations of the reagents can influence the reaction rate and product distribution.
- Temperature:Temperature can affect the reaction rate and selectivity, with higher temperatures typically favoring faster reactions but potentially decreasing selectivity.
- Solvent:The solvent can influence the solubility of the reactants and products, as well as the reaction rate and selectivity.
Examples of Successful Reagent Selection
In a recent study, the use of reagent 1 led to a significant improvement in the yield and selectivity of a reaction compared to reagent 2. The higher reactivity and selectivity of reagent 1 allowed for the efficient conversion of reactant A into product B with minimal side products.
Questions Often Asked
What are the key factors to consider when selecting reagents?
Reactivity, selectivity, and functional group compatibility are the primary factors to consider when selecting reagents.
How can I optimize reagent concentrations and reaction conditions?
Adjusting reagent concentrations and reaction conditions, such as temperature and solvent choice, can improve reaction yield and selectivity.
What are some examples of successful reagent selection?
Case studies and examples provided in the guide illustrate the practical application of reagent selection principles in achieving successful reaction outcomes.